Health
WHO Halts Malaria Drug Hydroxychloroquine Trial For Coronavirus Due To Safety Fears

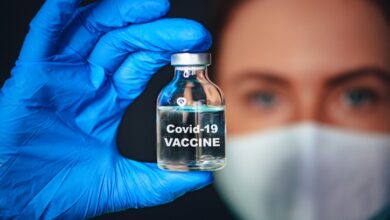
The World Health Organization (WHO) stopped testing of the malaria drug hydroxychloroquine as a possible treatment for coronavirus because of safety fears, reported the BBC.
On Monday, the WHO said trials in several countries are being “temporarily” suspended as a precaution. The announcement was made after a recent medical study suggested the drug could increase the risk of patients dying from Covid-19.
Tedros Adhanom Ghebreyesus, the WHO’s director-general, said the decision to halt the global clinical trial of hydroxychloroquine was taken in light of a Lancet observational study that claimed people taking hydroxychloroquine were at higher risk of death and heart problems as compared to those who were not.
“The executive group has implemented a temporary pause of the hydroxychloroquine arm within the solidarity trial while the safety data is reviewed by the data safety monitoring board,” Tedros said on Monday. “The other arms of the trial are continuing.”
The WHO is currently running clinical trials of various drugs to assess which might be beneficial in treating the disease. The international health body has previously raised concerns over reports of individuals self-medicating and causing themselves serious harm.
On Monday, the WHO officials said hydroxychloroquine would be removed from those trials pending a safety assessment.
The Lancet study involved over 96,000 coronavirus patients. Nearly 15,000 of the patients involved in the study were given hydroxychloroquine, or related form chloroquine, either alone or with an antibiotic. It was found that the patients were more likely to die in the hospital and develop heart rhythm complications than other COVID patients in a comparison group.
The death rates of the treated groups were hydroxychloroquine 18%; chloroquine 16.4%; control group 9%. Interestingly, those treated with hydroxychloroquine or chloroquine in combination with antibiotics had an even higher death rate. The study researchers warned that hydroxychloroquine should not be used outside of clinical trials.