South Africa
South African Government To Begin Coronavirus Vaccination Drive With J&J Vaccine
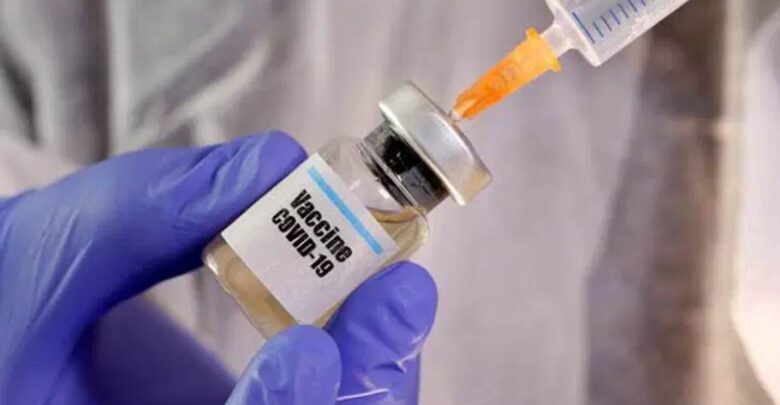
South African Health Minister Zweli Mkhize on Wednesday announced the government has decided to go ahead with its planned coronavirus vaccination campaign with the Johnson and Johnson vaccine, instead of the AstraZeneca/Oxford vaccine whose effectiveness has come under scanner, reported Africa News.
The South African health authorities suspended the start of the coronavirus vaccination program on Sunday after a study showed limited efficacy of the AstraZeneca/Oxford vaccine against the local variant of the virus, known as 501Y.V2.
The study claimed that the vaccine is only 22 percent effective against moderate forms of the South African coronavirus variant. No results are yet available on its efficacy against severe forms.
Given the results of the efficacy studies, (the government) will continue the planned first phase of vaccination using Johnson & Johnson vaccines instead of AstraZeneca vaccine,” Mkhize announced to the press.
However, he did not specify any date for the launch of the vaccination campaign.
He said the J&J vaccines will be used to launch the first phase of the vaccination drive in which the country’s 1.25 million healthcare workers will be inoculated. The one-shot J&J vaccine is still being tested internationally and has not been approved in any country.
Mkhize declared that the vaccine is safe, relying on tests of 44,000 people done in South Africa, the United States, and Latin America.
“The efficacy of the Johnson & Johnson vaccine against the 501Y.V2 variant has been proven,” he said.
The South African government will launch the roll-out of the vaccine in the form of an implementation study with the partnership of the Medical Research Council and the National Department of Health vaccination sites across the country.
South Africa has recorded almost 1.5 million coronavirus cases and over 46,000 deaths since the pandemic began – a higher toll than any other country on the continent.