Egypt
Egyptian Company To Start Manufacturing China’s Sinovac Covid-19 Vaccine In June
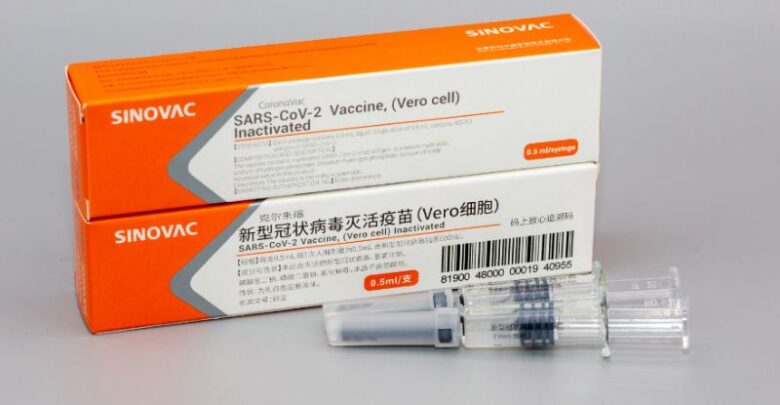
Egyptian Health Minister Hala Zayed on Sunday said the country will start local production of 60 million doses of the Sinovac COVID-19 vaccine next month, reported Ahram Online.
During a press conference in Cairo, Zayed said the first 2 million doses will be produced in June at the plants of the Egyptian Holding Company for Biological Products and Vaccines (VACSERA).
The Egyptian minister said Egypt will receive the first shipment of the material required for producing the final vaccine on May 18. The end-product will be made to undergo an assessment by the Egyptian Drug Authority (EDA) for few weeks before they become available for use.
VACSERA and the China-based Sinovac biopharmaceutical company signed two agreements last month to manufacture the vaccine so as to meet the country’s vaccination needs.
Under the first agreement, Sinovac will provide VACSERA the know-how and technical assistance for manufacturing the COVID-19 vaccine in Egypt. As per the second agreement, Sinovac will give VACSERA the license to manufacture, re-pack, and pack the COVID-19 vaccine in VACSERA facilities.
Zayed revealed that the locally produced Sinovac vaccine will be dubbed Sinovac-Vacsera and will have the Made in Egypt label.
The Egyptian health minister noted that it will later be fully manufactured in Egypt after implementing the agreement on transferring the manufacturing technology.
Egypt has reported 236,272 Covid-19 cases, including 13,845 deaths and 176,363 recoveries so far. The health authorities began the vaccination campaign in late January with a Covid-19 vaccine made by another leading Chinese drugmaker, Sinopharm, the first-approved vaccine by the Egyptian Drug Authority.
According to the Egyptian health ministry, about 900,000 people out of two million who had registered for vaccination have got vaccinated.
Egypt is scheduled to receive millions of coronavirus vaccine doses through the COVID-19 Vaccines Global Access (COVAX) and Sinopharm in the coming weeks.