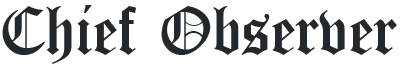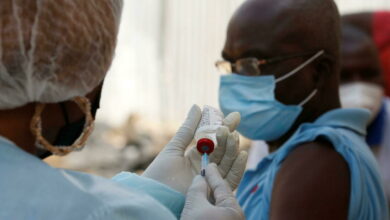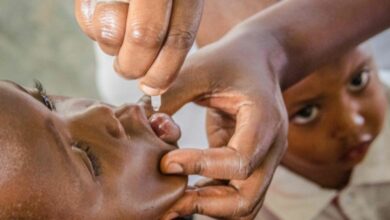
Scientists might have finally found the most effective drugs in treating Ebola in two of the four experimental drugs trialed in the Democratic Republic of the Congo, reported Reuters.
The two potential drugs- Regeneron’s REGN-EB3 and a monoclonal antibody called mAb114- showed survival rates of as much as 90 percent in the clinical trial which started in November last year. The two other drugs being tested are- ZMapp, made by Mapp Biopharmaceutical, and Remdesivir, produced by Gilead Sciences. The trial is being conducted by an international research group coordinated by the WHO.
The drugs were both developed using antibodies harvested from survivors of Ebola infection. They showed better results in treating Ebola patients in a trial out of four potential treatments being conducted in the DRC, which is currently facing the world’s second-largest Ebola outbreak in history. the outbreak has killed at least 1,800 people.
“Moving forward, these are the only drugs that future patients will be treated with,” the World Health Organization (WHO) said in a statement released on Monday.
Anthony Fauci, the director of the US National Institute of Allergy and Infectious Diseases and one of the researchers co-leading the trial, told reporters in a telebriefing that the clinical trial results were “very good news” for the fight against Ebola.
“What this means is that we do now have what look like treatments for a disease for which not long ago we really had no approach at all,” Fauci said.
He revealed that some 681 patients have already been enrolled in the clinical trial at four separate treatment centers in Congo. The study aims to enroll a total of 725.
Mike Ryan, head of the WHO’s emergencies programme, said the trial’s positive findings were encouraging but would not be enough on their own to put an end to the epidemic.